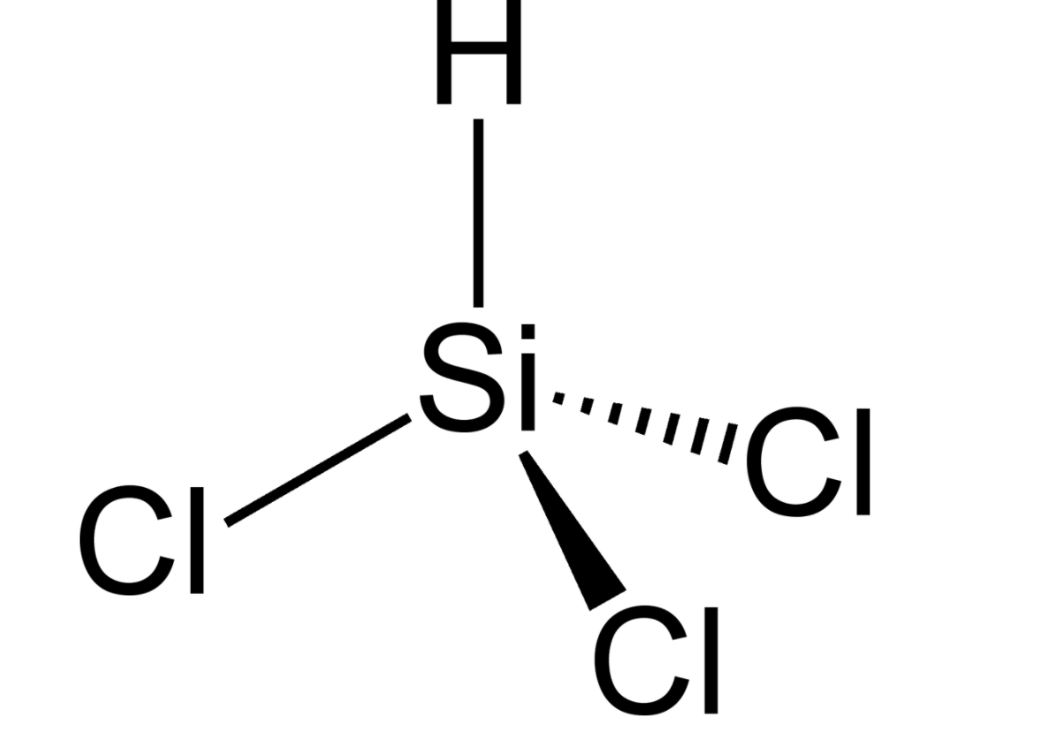
Trichlorosilane Market Risen Strongly
Typically, a polysilicon manufacturer needs to consume 0.5 tonnes of trichlorosilane for every extra tonne of poly-silicon capacity. This means that trichlorosilane is expected to fully benefit from the development of the PV industry. In the two-carbon context, capacity growth is rapid. The imbalance between supply and demand is driving prices up. According to data, photovoltaic grade trichlorosilane prices have risen by more than 170%. On the supply side,trichlorosilane capacity is not scarce. But for photovoltaic grade trichlorosilane products, the production is difficult and the capacity is seriously insufficient. In addition, as the second largest trichlorosilane coupling agent in the downstream market of trichlorosilane (40%). As automotive lightweight has become a mainstream trend, the demand for trichlorosilane coupling agents has grown steadily in recent years.
Trichlorosilane (TCS or SiHCl3) is generated as follows within a high temperature, pressurized reactor: Si + 3 HCl ➡ SiHCl3 + H2 ,Si + 3 SiCl4 +2 H2 ➡ 4 SiHCl3 .The TCS is then sent to the CVD (Chemical Vapor Desposition) reactor. In the Siemens process, high-purity silicon "starter" rods or hairpins are exposed to trichlorosilane at 1150 °C in the CVD reactor. The trichlorosilane gas decomposes and deposits additional pure silicon onto the rods in the CVD reactor, enlarging them according to chemical reactions like: 2 SiHCl3 + H2 ➡ Si + SiCl4 + 2 HCl (at 1100 °C / 6 barg) Silicon produced in this reaction is called polycrystalline silicon (PCS). The SiCl4 + HCl is then "recycled" and returned to the TCS plant via SiCl4 conversion (in the converter): SiCl4 +H2 + catalyst ➡ HCl + SiHCl3 .
In all of the above reactions, we note the use or generation of HCl and H2. Make-up anhydrous HCl gas is needed in the TCS reactor due to the incomplete conversion and recycling of the SiCl4 and TCS in the recycling step, meaning that some of the chlorine is generated as a waste product in the overall process and sent elsewhere. The AHCl needs to be dry and of high purity but it can contain H2 , which is also a recycled bi-product.