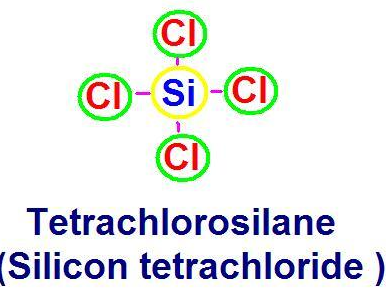
SiCl4 Silicon tetrachloride
Silicon tetrachloride, SiCl4 Silicon tetrachloride is a simple no-messing-about covalent chloride. There isn’t enough electronegativity difference between the silicon and the chlorine for the two to form ionic bonds. Silicon tetrachloride is a colourless liquid at room temperature which fumes in moist air. These ions are mobile hence they conduct electricity. carbon tetrachloride and silicon tetrachloride have no mobile ions and therefore they can’t conduct electricity.
In recent years, China's polysilicon production has shown geometric development, but the disposal of polysilicon by-product silicon tetrachloride has become a difficult to step over the development of polysilicon industry "sill", let the silicon industry wear the hat of high pollution. According to industry production estimates, the improved process produces 1 ton of polysilicon for every 10 to 15 tons of silicon chloride. This means that at least one million tonnes of silicon tetrachloride are produced annually.
How to deal with and utilize a large number of by-products effectively and reduce environmental pollution has become a common problem faced by polysilicon production enterprises. In order to reduce environmental pollution and production cost, silicon tetrachloride should be rationally utilized.