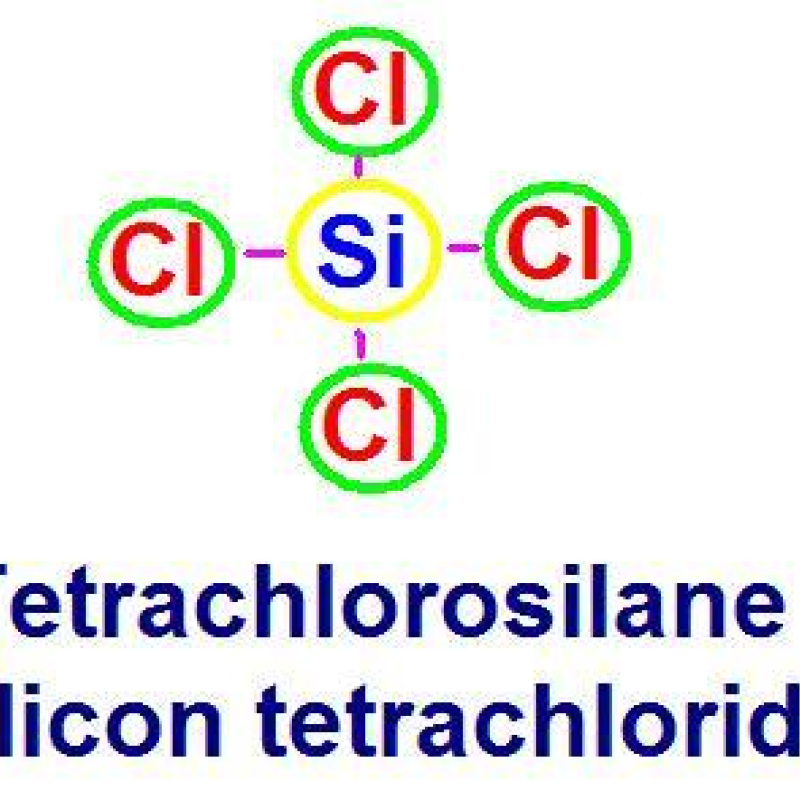
Silicon Tetrachloride Low Temperature Hydrogenation Technology
Low temperature hydrogenation technology was a technology to hydrogenate silicon tetrachloride with silicon powder and catalyst at a lower reaction temperature. Using copper or iron based catalyst, at the temperature of 400 ~ 800℃, pressure of 2 ~ 4MPa, adding silicon powder and hydrogen to the fluidized bed and reacting with silicon tetrachloride to produce trichlorosilane. The basic reaction principle of low-temperature hydrogenation is as follows:
3SiCl4(g)+2H2(g)+Si(s)=4SiHCl3(g)
Chlorine hydrogenation technology is to add HCl on the basis of low temperature hydrogenation technology to further reduce the reaction temperature and increase the yield of trichlorosilane. Chlorine hydrogenation reaction principle is as follows: 2SiCl4(g)+H2(g)+HCl(g)+Si(s)=3SiHCl3(g). Hydrogen plasma is generated by hydrogen discharge, which is passed into the reactor to react with silicon tetrachloride gas. Since hydrogen is dissociated into hydrogen atoms, the reactivity is greatly increased and it can easily react with silicon tetrachloride to form trichlorosilane. Catalytic hydrogenation is a technology to produce trichlorosilane by passing a mixture of silicon tetrachloride and hydrogen through a molecular sieve loaded with a catalyst. Reaction principle is as follows: SiCl4(g)+H2(g)SiHCl3(g)+HCl(g).