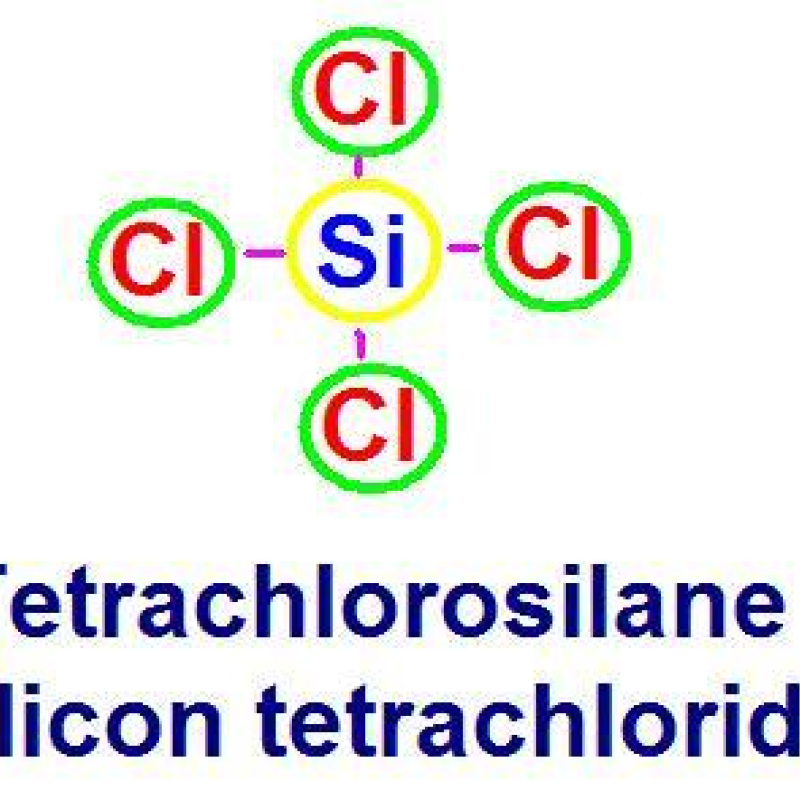
Silicon Tetrachloride
The application of silicon tetrachloride is very wide, involving military, metallurgy, industry, integrated circuit, photovoltaic, optical fiber and other fields, its market demand is large. At the same time, with the upgrading of the market demand for silicon tetrachloride and the development of the fiber prefabricated rod industry, the market demand for high-purity fiber grade silicon tetrachloride has increased significantly, and there is a broad space for domestic substitutes. The market size of silicon tetrachloride in China is expected to reach over one billion yuan by 2022.
The basic principle of solid adsorption is adsorption separation based on the different polarity of chemical bonds of each component in a compound. Silicon tetrachloride is a symmetric molecule with no dipole moment. In contrast, the contained impurities such as BCl3, AlCl3, FeCl3, PCl3, etc. are asymmetric molecules with considerable dipole moments, which strongly tend to form additive chemical bonds and are easily adsorbed by the adsorbent. In addition, the surface of adsorbents (such as silica gel, etc.) is covered by hydroxide groups, so it is easy to adsorb ionic compounds and easily hydrolyzed compounds.
Solid adsorption method can overcome the difficulty of removing strong polar impurities by rectification method. The adsorbents effective for silicon tetrachloride include various activated carbon, hydrated oxide and silicate, among which activated alumina and silica gel have the best effect. In the adsorption operation, it is the key to prepare ultra-pure adsorbent and avoid contamination.