At present, amine salt type corrosion inhibitors are widely used and have superior effects. Through physical and chemical analysis of the sample, ultraviolet spectrum, infrared spectrum, nuclear magnetic resonance spectroscopy and ion chromatography and other series of analysis and prediction to determine the role of different ions of dicyclohexylamine salt corrosion inhibitor in steel corrosion analysis, and finally determined that the solid sample may be dicyclohexylamine nitrite.
Contact Now
A vapor phase corrosion inhibitor preparation method, including the following steps :a. Dispersing montmorillonite in deionized water, beating, stirring, and then taking the suspension of the solution to add morpholine, heating the solution to evaporate and precipitate crystals.b. Add formaldehyde to the solution in Step a and let it stand; add dicyclohexylamine and let it stand; then add the mixed solution of benzoic acid and acetone to the solution and let it stand to generate white slurry.
Contact Now
Cyclohexylamine (CHA) is a primary amine, can be miscible with water with infinite ratio, the molecule is small, easy to adsorb on the metal surface, its shielding effect is better than long chain primary amine and secondary amine, so it can effectively prevent the invasion of corrosive ions, thereby improving corrosion resistance, especially the ability to inhibit corrosion.The corrosion inhibition of 304 stainless steel by cyclohexylamine in sodium chloride medium was studied in detail.
Contact Now
The invention relates to a synthesis method of dicyclohexylamine, which comprises the following steps: in a hydrogenation reactor, aniline and hydrogen are hydrogenated in gas phase under the catalyst to obtain DCHA crude product.The replacement reaction was carried out with aniline and DCHA crude product to obtain the replacement mother liquor. The replacement mother liquor was delight and refined, and DCHA product was obtained at the top of the refining tower.
Contact Now
DCHA at home and abroad is usually a by-product of CHA production. In the process of CHA synthesis by aniline hydrogenation, about 10% of DCHA can be obtained by by-product. In addition, according to the different catalysts, the yield of DCHA prepared by aniline hydrogenation can reach up to 70%, and the yield of cyclohexylamine (CHA) is 30%.Benzene, cyclohexane, cyclohexanol, N-cyclohexylcyclohexamine, phenylcyclohexylamine and other by-products will be generated in the reaction.
Contact Now
Cyclohexylamine has the chemical properties of a primary amine. Carbonates that are strongly alkaline and absorb carbon dioxide in the air to form white crystals. It can react with acyl chloride, anhydride and ester to form N-acyl. React with nitrite to give alcohol. Hydroxymethyl compounds are formed by reaction with formaldehyde in alkaline solution. Reacts with carbon disulfide to form dithiocarbamate. Reacts with aldehydes to form Schiff bases.This product is used to prepare cyclohexanol, caprolactam, acetate fiber and nylon 6, etc.
Contact Now
The method of species co-production of cyclohexylamine and dicyclohexylamine and the catalyst system used for the method. The method includes the following steps :(1) the raw material aniline, cyclohexanone, ammonia, hydrogen after the loading load RhNi catalyst first section reactor, the first reaction liquid.(2) The reaction solution containing cyclohexylamine and dicyclohexylamine was obtained through the second stage reactor loaded with RhCo catalyst.
Contact Now
A sulfonate of cyclohexylamine; used as an artificial flavoring in food, beverages, and medicine. Also used as an acid gas absorber. Used as a pH regulator for boiler feed water. Cyclohexylamine is a volatile substance that can easily reach the whole system after dosing.If pH is lower than 8.5, the treatment effect of cyclohexylamine is not good. Synthetic desulfurizer, rubber promoter, dye, antistatic agent, corrosion inhibitor, pesticide fungicide, insecticide and other intermediates. Used as boiler water treatment agent, corrosion inhibitor, rubber promoter, etc.
Contact Now
Cyclohexylamine, an organic compound with the chemical components of c6h13n, is a drab to yellow liquid, soluble in water and miscible in most organic solvents. It is mainly used as a solvent, and can also be used to prepare desulfurizer, rubber antioxidant, vulcanization accelerator (CZ), cyclamate, medical raw materials, chemical additives for plastics and textiles, boiler feed water treatment agent, metal corrosion inhibitor, emulsifier, preservative, antistatic agent and latex coagulant.
Contact Now
The mixture of trichlorosilane and polysilicon, which is a by-product of the recombination from the bottom of the distillation column in the production of trichlorosilane and polysilicon, was used as raw material to prepare silica by gas phase hydrolysis. The effects of gasification temperature, flow rate and flow ratio of water vapor and chlorosilane mixture on physicochemical properties of the products were investigated.
Contact Now
To explore the application value of ion chromatography in the detection of cyclohexylamine in workplace air. Workplaces containing cyclohexylamine in the production of raw materials were selected as sampling sites, and the content of cyclohexylamine in workplaces was detected by ion chromatography. At the same time, the precision and recovery rate of ion chromatography, desorption efficiency, sampling efficiency and other cation interference characteristics were analyzed.
Contact Now
The production process of trichlorosilane is mostly prepared by reaction of gold-grade silicon metal powder and hydrogen chloride gas in a fluidized bed reactor. Generally using gold grade metal silicon powder, hydrogen chloride gas by the combustion reaction of chlorine and hydrogen gas. Reaction temperature is 300-400 degrees, most of the pressure using micro positive pressure operation.
Contact Now
The invention uses nitro-benzene and hydrogen as raw materials. Adding solvent, nitro-benzene and catalyst into the reaction kettle, controlling the reaction pressure of 0.5 ~ 4MPa, reaction temperature of 60 ~ 160℃, reaction for 2 ~ 8h, cyclohexylamine and dicyclohexylamine are obtained, and the catalyst used is Pd/CNTs catalyst or PD-Ni /CNTs catalyst.
Contact Now
Cyclohexylamine, an organic compound with the chemical formula of C6H13N, is a colorless to yellow liquid, soluble in water and miscible in most organic solvents. UsageIt is mainly used as solvent, and can also be used to prepare desulfurizer, rubber antioxidant, vulcanization accelerator, chemical additives for plastics and textiles, boiler feed water treatment agent, metal corrosion inhibitor, emulsifier, preservative, antistatic agent, latex coagulant, petroleum additive, bactericide, insecticide and dye intermediate.SpecificationProjectItemBest gradeFirst grade
Contact Now
At present, there are few reports on the quantitative analysis of dicyclohexylamine and impurities, so it is necessary to study them separately accurate quantitative analysis method. Since dicyclohexylamine has no ultraviolet absorption, the quantitative analysis method of dicyclohexylamine by high performance liquid chromatography evaporative light scattering detector was studied.
Contact Now
Since May 2022, trichlorosilane has been sustained upward by the downward trend in silicon metal prices.
Contact Now
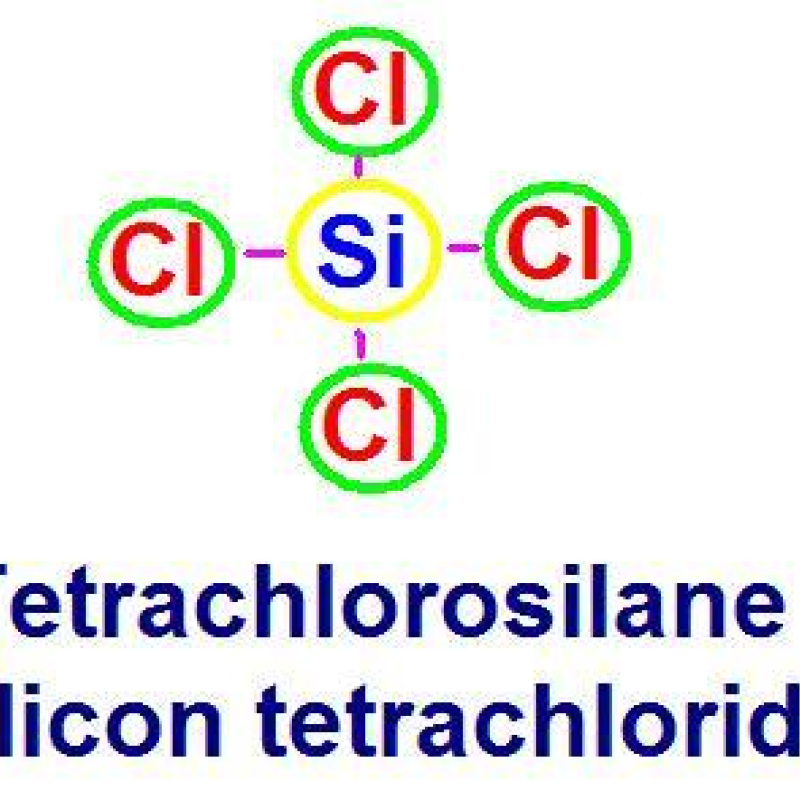
Silicon tetrachloride which chemical formula is SiCl4 is an inorganic compound, because silicon chloride only exists in the form of SiCl4, silicon tetrachloride is also directly called silicon chloride. The appearance of this product is light yellow liquid, and the appearance of high-purity silicon tetrachloride is colorless and transparent liquid, volatile, with special odor, toxicity and corrosion. Silicon tetrachloride can be miscible with benzene, chloroform, ether, carbon tetrachloride and other organic solvents. It can be hydrolyzed in humid air and react violently with water.
Contact Now
It is obtained by catalytic reduction of nickel or cobalt from aniline at high temperature and pressure. Cyclohexanol can also be reduced by phenol, oxidized to cyclohexanone, and then aminated with ammonia to obtain cyclohexanamine.This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies. Acute poisoning may occur by inhalation of vapour. Irritant and corrosive to skin, eyes and mucous membranes, and can cause allergy through skin absorption.
Contact Now
It is obtained by catalytic hydrogenation of aniline and can be divided into atmospheric pressure method and pressure method. In addition, cyclohexamine can be prepared by catalytic ammonolysis of cyclohexane or cyclohexanol, reduction of nitrocyclohexane, and catalytic ammonolysis of cyclohexanone in the presence of hydrogen.The refining process often contains impurities such as aniline and water.
Contact Now
In recent years, China's polysilicon production has shown geometric development, but the disposal of polysilicon by-product silicon tetrachloride has become a difficult to step over the development of polysilicon industry "sill", let the silicon industry wear the hat of high pollution.
Contact Now
The basic principle of solid adsorption is adsorption separation based on the different polarity of chemical bonds of each component in a compound. Silicon tetrachloride is a symmetric molecule with no dipole moment. In contrast, the contained impurities such as BCl3, AlCl3, FeCl3, PCl3, etc. are asymmetric molecules with considerable dipole moments, which strongly tend to form additive chemical bonds and are easily adsorbed by the adsorbent.
Contact Now
A method of cyclohexylamine catalytic production of biodiesel with prickly ash seed oil as raw material is described as follows: prickly ash seed oil is mixed with methanol and the catalyst cyclohexylamine.
Contact Now
By controlling the parameters in each step, such as time, temperature, pressure, etc.The yield of cyclohexylamine products is high, the product yield is more than 99%, the quality is stable, the yield is high, the production capacity is large, the side reaction is less, and a small amount of by-product dicyclohexylamine in the reaction can be recovered and purified, and the added value of the products can be increased.
Contact Now
The principle is to reduce high purity trichlorosilane with high purity hydrogen on the high purity silicon core at about 1100℃ to generate polycrystalline silicon deposited on the silicon core. On the basis of the traditional Siemens process, the improved Siemens process is equipped with a supporting process of energy saving, consumption reduction, recycling and utilization of a large amount of H2, HCI, SiCI4 and other by-products and a large amount of by-production heat energy.
Contact Now