In silicon tetrachloride purification, boron is the most difficult impurity to remove. Researchers have proposed compound method to remove boron from silicon tetrachloride, which has achieved satisfactory results and has been widely used in industrial production.
Contact Now
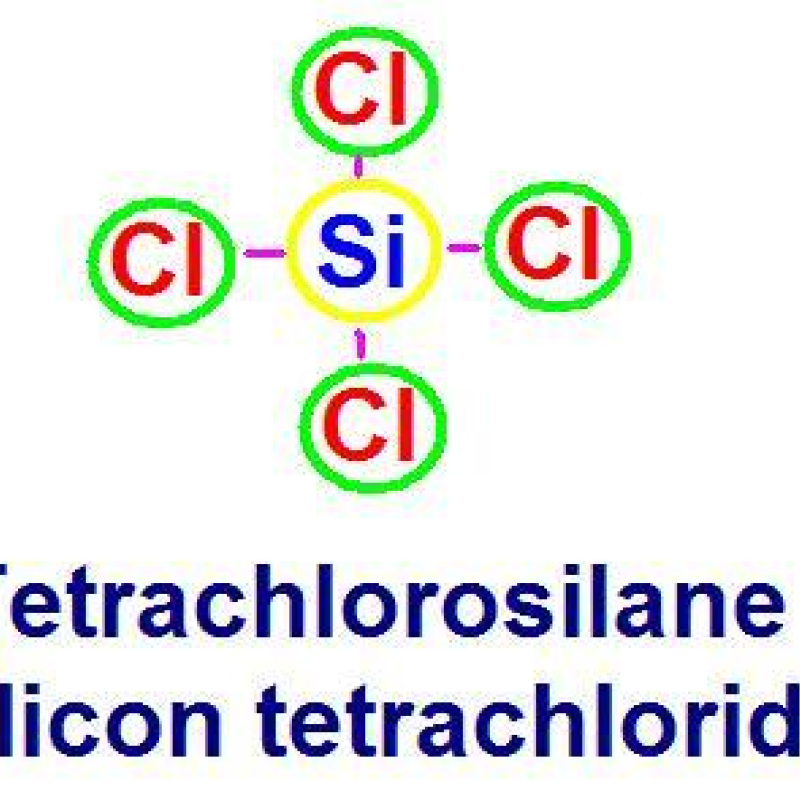
Silicon tetrachloride which chemical formula is SiCl4 is an inorganic compound, because silicon chloride only exists in the form of SiCl4, silicon tetrachloride is also directly called silicon chloride. The appearance of this product is light yellow liquid, and the appearance of high-purity silicon tetrachloride is colorless and transparent liquid, volatile, with special odor, toxicity and corrosion. Silicon tetrachloride can be miscible with benzene, chloroform, ether, carbon tetrachloride and other organic solvents. It can be hydrolyzed in humid air and react violently with water.
Contact Now
In recent years, China's polysilicon production has shown geometric development, but the disposal of polysilicon by-product silicon tetrachloride has become a difficult to step over the development of polysilicon industry "sill", let the silicon industry wear the hat of high pollution.
Contact Now
The basic principle of solid adsorption is adsorption separation based on the different polarity of chemical bonds of each component in a compound. Silicon tetrachloride is a symmetric molecule with no dipole moment. In contrast, the contained impurities such as BCl3, AlCl3, FeCl3, PCl3, etc. are asymmetric molecules with considerable dipole moments, which strongly tend to form additive chemical bonds and are easily adsorbed by the adsorbent.
Contact Now
Silicon tetrachloride is an important chemical raw material, it is widely used to produce high-purity silicon, silane, silicone ester, silicone oil, silica gel and other products, widely used in ink, paint, resin, rubber, medicine, grease and other fields; Silicon tetrachloride can also be used to manufacture optical fiber, polysilicon, silicon dioxide, etc., in communications, photovoltaic power generation, optical instruments and other fields of application; In addition, silicon tetrachloride can also be used in the military field, metallurgy, casting and other fields.
Contact Now
Distillation is the use of SiCl4, and the difference between the relative volatility of impurities components, through several partial gasification and partial condensation process, to achieve the separation of mixed liquid, so as to obtain high purity silicon tetrachloride products.
Contact Now
The main by-product of trichlorosilane, silicon tetrachloride, is also the main raw material for the manufacture of silicone, its finished products are silicone ester, silicone oil, high temperature insulating paint, silicone resin, silicone rubber and heat resistant cushion lining materials. High purity silicon tetrachloride is also an important raw material for manufacturing high purity silicon dioxide, inorganic silicon compounds, quartz fibers and optical fibers.
Contact Now
Silicon tetrachloride is mainly used in the manufacture of silicone esters, gaseous silica, silicone monomer, silicone oil, high temperature insulating paint, silicone resin, silicone rubber and so on. High-purity silicon tetrachloride is the main raw material for manufacturing fiber prefabricated rod, and the quality of fiber prefabricated rod directly determines the fiber performance. Therefore, high-purity silicon tetrachloride is the core raw material of fiber industry.
Contact Now
SiCl4 is one of the most important inorganic silicon compounds, is a colorless, transparent, flowing smoke liquid, with suffocating odor, soluble in benzene, ether, chloroform and other most organic solvents. Silicon tetrachloride is a volatile liquid with strong asphyxiating odor. It can be used as raw materials for the production of gaseous silica, high purity silicon and organosilicide. The upstream of the silicon tetrachloride industry chain is the raw material market, mainly silicon powder, ferrosilicon, hydrogen chloride, etc.
Contact Now
Chlorine hydrogenation technology is to add HCl on the basis of low temperature hydrogenation technology to further reduce the reaction temperature and increase the yield of trichlorosilane. Chlorine hydrogenation reaction principle is as follows: 2SiCl4(g)+H2(g)+HCl(g)+Si(s)=3SiHCl3(g). Hydrogen plasma is generated by hydrogen discharge, which is passed into the reactor to react with silicon tetrachloride gas. Since hydrogen is dissociated into hydrogen atoms, the reactivity is greatly increased and it can easily react with silicon tetrachloride to form trichlorosilane.
Contact Now
High temperature hydrogenation of silicon tetrachloride is an important method to treat silicon tetrachloride as a by-product of polysilicon. High temperature hydrogenation is silicon tetrachloride and hydrogen as raw materials, heated by 1200 ~ 1250℃ graphite heater, thermal reduction reaction to produce trichlorosilane. The advantages of the process are that the whole system is closed circulation, suitable for continuous and stable operation; Trichlorosilane products of high purity, need to distillation links less.
Contact Now
Silicon tetrachloride is an important raw material and intermediate product in the process of organic silicon synthesis and polysilicon production. Under different environmental conditions, silicon tetrachloride can react with a variety of substances to produce new substances.
Contact Now
Colorless liquid with stinky smell. it will decompose with water and dissolve in carbon disulfide, carbon tetrachloride, chloroform, benzene, and many others. flammable, can spontaneously ignite inside the air. poisonous!SpecificationInChIKeyZDHXKXAHOVTTAH-UHFFFAOYAHUN number1295RTECSVV5950000Chemical FormulaSiHCl3Mole Mass135.4524 g·mol⁻¹Exteriorcolourless liquidDensity1.342 g/cm3Melting Point-126.6 °CBoiling Point31.8 °CSolubility (water)Decomposition by waterVapour Pressure0.660 bar (20 °C)It can undergo an addition reaction with olefins.
Contact Now
Colorless liquid with pungent odor. It will decompose with water and dissolve in carbon disulfide, carbon tetrachloride, chloroform, benzene, etc. Flammable, can spontaneously ignite in the air. Poisonous! SpecificationInChIKeyZDHXKXAHOVTTAH-UHFFFAOYAHUN number1295RTECSVV5950000Chemical FormulaSiHCl3Mole Mass135.4524 g·mol⁻¹Exteriorcolourless liquidDensity1.342 g/cm3Melting Point-126.6 °CBoiling Point31.8 °CSolubility (water)Decomposition by waterVapour Pressure0.660 bar (20 °C)
Contact Now
Colorless liquid with smelly odor. it'll decompose with water and dissolve in carbon disulfide, carbon tetrachloride, chloroform, benzene, and plenty of others. flammable, can spontaneously ignite within the air. toxic!It could go through an addition response with olefins.The hydrosilylation response of ethylene is as follows: cl3si-h + h2c=ch2 → cl3si-ch2-ch3 .
Contact Now
Colorless liquid with stinky scent. it's going to decompose with water and dissolve in carbon disulfide, carbon tetrachloride, chloroform, benzene, and lots of others. flammable, can spontaneously ignite in the air. poisonous!It may undergo an addition reaction with olefins.The hydrosilylation reaction of ethylene is as follows: cl3si-h + h2c=ch2 → cl3si-ch2-ch3 .
Contact Now
Cyclohexylamine, an organic compound with the chemical components of c6h13n, is a drab to yellow liquid, soluble in water and miscible in most organic solvents. It is mainly used as a solvent, and can also be used to prepare desulfurizer, rubber antioxidant, vulcanization accelerator (CZ), cyclamate, medical raw materials, chemical additives for plastics and textiles, boiler feed water treatment agent, metal corrosion inhibitor, emulsifier, preservative, antistatic agent and latex coagulant.
Contact Now
At present, there are few reports on the quantitative analysis of dicyclohexylamine and impurities, so it is necessary to study them separately accurate quantitative analysis method. Since dicyclohexylamine has no ultraviolet absorption, the quantitative analysis method of dicyclohexylamine by high performance liquid chromatography evaporative light scattering detector was studied.
Contact Now
Cyclohexylamine, an organic compound with the chemical formula of C6H13N, is a colorless to yellow liquid, soluble in water and miscible in most organic solvents. UsageIt is mainly used as solvent, and can also be used to prepare desulfurizer, rubber antioxidant, vulcanization accelerator, chemical additives for plastics and textiles, boiler feed water treatment agent, metal corrosion inhibitor, emulsifier, preservative, antistatic agent, latex coagulant, petroleum additive, bactericide, insecticide and dye intermediate.SpecificationProjectItemBest gradeFirst grade
Contact Now
Dicyclohexylamine has the chemical properties of a secondary amine. It has a strong base and can form salts with various acids. Acylation may occur. In terms of solubility, dicyclohexylamine is slightly soluble in cold water but almost insoluble in hot water. Dry powder, carbon dioxide, soluble foam and sand are used to extinguish the fire.
Contact Now
The process relates to a tail gas refining method, in particular to the hydrogenation of aniline to prepare cyclohexylamine and the deamination of dicyclohexylamine tail gas to refine hydrogen.
Contact Now
A sulfonate of cyclohexylamine; used as an artificial flavoring in food, beverages, and medicine. Also used as an acid gas absorber. Used as a pH regulator for boiler feed water. Cyclohexylamine is a volatile substance that can easily reach the whole system after dosing.If pH is lower than 8.5, the treatment effect of cyclohexylamine is not good. Synthetic desulfurizer, rubber promoter, dye, antistatic agent, corrosion inhibitor, pesticide fungicide, insecticide and other intermediates. Used as boiler water treatment agent, corrosion inhibitor, rubber promoter, etc.
Contact Now
It is obtained by catalytic reduction of nickel or cobalt from aniline at high temperature and pressure. Cyclohexanol can also be reduced by phenol, oxidized to cyclohexanone, and then aminated with ammonia to obtain cyclohexanamine.This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies. Acute poisoning may occur by inhalation of vapour. Irritant and corrosive to skin, eyes and mucous membranes, and can cause allergy through skin absorption.
Contact Now
It is obtained by catalytic hydrogenation of aniline and can be divided into atmospheric pressure method and pressure method. In addition, cyclohexamine can be prepared by catalytic ammonolysis of cyclohexane or cyclohexanol, reduction of nitrocyclohexane, and catalytic ammonolysis of cyclohexanone in the presence of hydrogen.The refining process often contains impurities such as aniline and water.
Contact Now